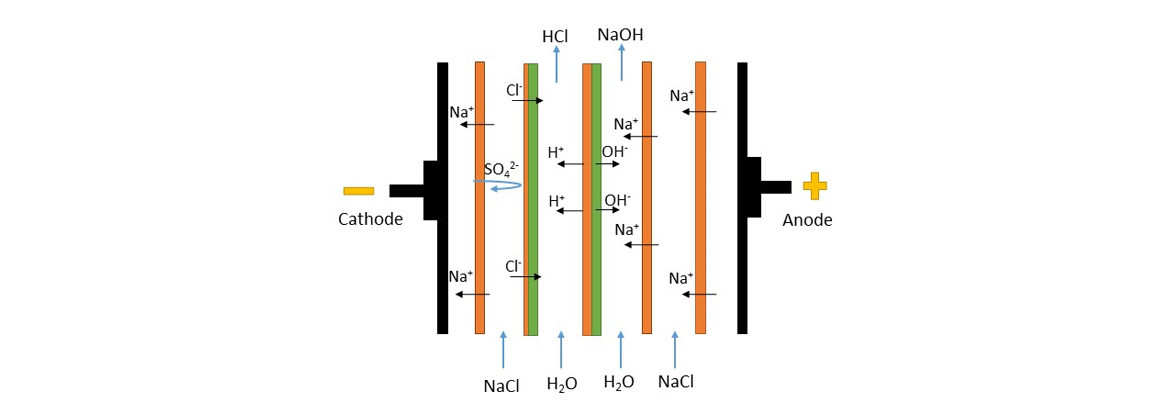
Description:
Bipolar membrane electrodialysis (BMED) is a technique based on the use of these membranes, capable of generating protons (H+) and hydroxyl ions (OH–) from electric current. Concentrated acids and bases can be obtained from salt solutions with a lower energy input than that required in other techniques. This type of technology, unlike metathesis, does not aim to recover more water, but to obtain second-generation products or by-products derived from brine. It aims to value brine, being fully involved in the circular economy of the region in the that is available. Bipolar membranes are an evolution of those used in conventional electrodialysis, made up of a cation exchange membrane and an anion exchange membrane, separated by a porous medium. This intermediate region is called the transition region or interface.
These membranes allow the dissociation of water molecules with an electric field, generating protons (H+) and hydroxyls (OH–) on the cationic and anionic side, respectively. Subsequently, the protons and the hydroxyls react with the other ions present in the water, generating the acids and bases discussed above. Note the equations:
H2O à H+ + OH–
X–+ H+ à HX
M++ OH– à MOH
The arrangement of the homopolar and bipolar membranes will vary depending on the application. The following figure shows the configuration used to recover products from a desalination plant brine. In this case it has a monoionic membrane so that SO42- cannot pass, obtaining a hydrochloric acid that is as pure as possible.
Objetive:
Obtaining concentrated acids and bases as subproducts from saline solutions.
Type of water:
Brine from brackish water desalination.
Brine from seawater water desalination.
Saline wastewater.
Applications:
Brine treatment
Recovery of second-generation products
Reuse of industrial wastewater for the production of acids and bases
Comparison to established technologies:
Advantages:
Absence of applied pressure.
Lower fouling potential due to lack applied pressure.
Multiple application.
Environmentally friendly technology
Disadvantages:
Technology highly used in other types of industries such as food. It is necessary to carry out more studies with the use of brines in terms of cost and energy consumption.
Applicability:
TRL 4 in 2021
Technological readiness level (TRL), it is an indicator of maturity of a particular technology. TRL is measured on a scale from 1 to 9, 1 being the lowest and 9 being the highest. A TRL of 9 reflects the implementation of an actual system in operational environment.
J.C. Mankins, Technology Readiness Levels. White Paper, April, 1995 https://www.spacepropulsion.org/uploads/2/5/3/9/25392309/john_mankins_paper_of_4-6-95_trl.pdf
Other issues:
Reverse Osmosis (RO) – Bipolar membranes electrodialysis (BMED)
Nanofiltration – Bipolar membranes electrodialysis with (BMED) for the production of soda from brine.
Selectrodialysis and bipolar membrane electrodialysis combination for industrial process brines treatment.
Energy consumption:
Specific energy consumption, as reported in literature varies depending on feed water concentrations (1.6-9 kWh/Kg of acid).
Herrero-Gonzalez, M., Diaz-Guridi, P., Dominguez-Ramos, A., Irabien, A., & Ibañez, R. (2020). Highly concentrated HCl and NaOH from brines using electrodialysis with bipolar membranes. Separation and Purification Technology, 116785.
Research directions:
Using nanocomposite anion exchange membranes in order to reduce the sulfate content as the main impurity in the acid stack.
Carbon dioxide capture coupled with magnesium utilization from seawater
by bipolar membrane electrodialysis.
Data Sources:
[1] Tongwen, X. (2002). Electrodialysis processes with bipolar membranes (EDBM) in environmental protection—a review. Resources, conservation and recycling, 37(1), 1-22.
[2] Medina Collana, J., & Pantoja Cadillo, A. (2013). Evaluación de parámetros de operación de un módulo de electrodiálisis con membrana bipolar en la producción de hidróxido de sodio a partir de aguas residuales por osmosis inversa.
[3] Valverde Estrella, E. (2014). Obtención de ácidos y bases a partir de disoluciones salinas concentradas mediante electrodiálisis con membranas bipolares.
[4] Wang, M., Wang, K. K., Jia, Y. X., & Ren, Q. C. (2014). The reclamation of brine generated from desalination process by bipolar membrane electrodialysis. Journal of membrane science, 452, 54-61.
[5] Parra Carmona, A. (2015). Valorización de salmueras concentradas mediante electrodiálisis con membranas bipolares (Bachelor’s thesis, Universitat Politècnica de Catalunya).
[6] Reig, M., Casas, S., Gibert, O., Valderrama, C., & Cortina, J. L. (2016). Integration of nanofiltration and bipolar electrodialysis for valorization of seawater desalination brines: production of drinking and waste water treatment chemicals. Desalination, 382, 13-20.
[7] Fernandez-Gonzalez, C., Dominguez-Ramos, A., Ibañez, R., Chen, Y., & Irabien, A. (2017). Valorization of desalination brines by electrodialysis with bipolar membranes using nanocomposite anion exchange membranes. Desalination, 406, 16-24.
[8] Herrero-Gonzalez, M., Diaz-Guridi, P., Dominguez-Ramos, A., Irabien, A., & Ibañez, R. (2020). Highly concentrated HCl and NaOH from brines using electrodialysis with bipolar membranes. Separation and Purification Technology, 116785.
[9] Li, F. R., Jia, Y. X., Guo, R. Q., & Wang, M. (2021). Preparation of composite anion-exchange membrane with acid-blocking performance for brine reclamation by bipolar membrane electrodialysis. Separation and Purification Technology, 254, 117587.
[10] Tianyi Chen, Jingtao Bi, Yingying Zhao, Zhongte Du, Xiaofu Guo, Junsheng Yuan, Zhiyong Ji, Jie Liu, Shizhao Wang, Fei Li, Jing Wang (2022). Carbon dioxide capture coupled with magnesium utilization from seawater by bipolar membrane electrodialysis, Science of The Total Environment, Volume 820,153272.