Description:
Adsorption desalination (AD) is a phase transfer process that occurs at the interface between a solid and an aqueous phase where salt ions (the adsorbate) adsorb on the surface of porous solids (the adsorbent) via physical interactions (such as van der Waals interaction and hydrogen bonding) and/or chemical bonding. Adsorption can be reversed, i.e., desorption, especially physical adsorption, to regenerate the adsorbent, which is key for the development of practical processes.
The adsorption desalination (AD) cycle works on two main processes:
(i) the adsorption-evaporation process and (ii) the desorption-condensation process.
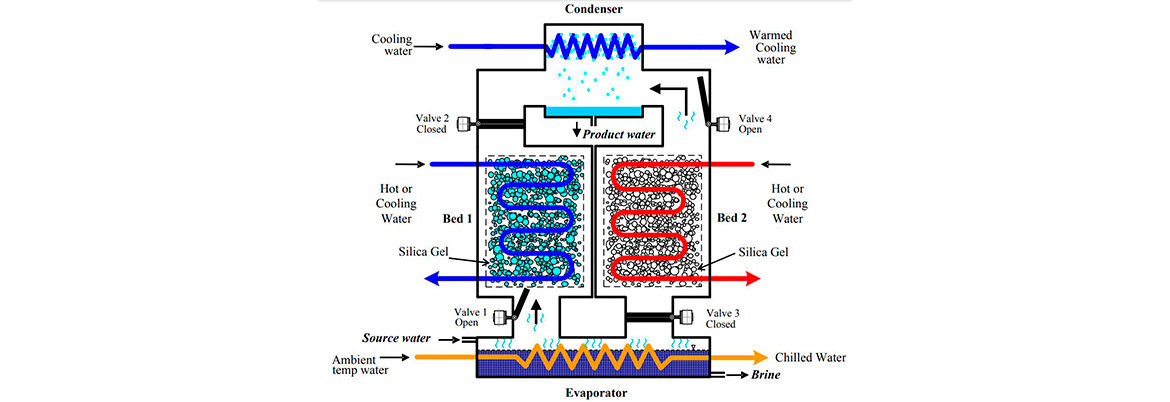
A schematic diagram of a typical two-bed AD in shown in figure 1. The system consists of three main components: the evaporator, the adsorption bed and the condenser.
Adsorption desalination is a phase transfer process that occurs at the interface between a solid and an aqueous phase where salt ions (the adsorbate) adsorb on the surface of porous solids (the adsorbent) via physical interactions (such as van der Waals interaction and hydrogen bonding) and/or chemical bonding. Adsorption can be reversed, i.e., desorption, especially physical adsorption, to regenerate the adsorbent, which is key for the development of practical processes.
Figure 1 shows a schematic of a two-bed adsorption-based desalinator, which is the most basic model of any practicable production system (i.e. one that is capable of producing fresh water on an essentially continuous manner). This system consists of three major components: a condenser, two silica gel beds, and an evaporator. The source saline water is first charged into the evaporator and a vacuum is applied to the entire system. Valve 1 is then opened to allow the source water to evaporate at temperature Tevap and travel as a vapour into Bed 1 where it adsorbs on the silica gel. The heat generated during adsorption is removed by cold water circulating at temperature Tcw in Bed 1. Once the silica in Bed 1 is saturated with water, Valve 1 is closed. The water circulating through Bed 1 is then switched to hot water at temperature Thw to bring the bed pressure up to the condenser pressure. Valve 2 is then opened so as to allow the adsorbed water in Bed 1 to pass into the condenser to form, finally, pure water at a temperature Tcond. Once much if not all the water has been driven off the silica gel (the amount depends on the hot water temperature), this silica gel regeneration process stops. Valve 2 is then closed and cold water (at Tcw) is circulated through Bed 1 to reduce its pressure to the evaporator pressure. Valve 1 is then opened to start the next cycle. The time elapsed from the beginning of the process until now is so called cycle time. Pure water is produced, essentially, continuously by the system in Figure 1. by carrying out the above process alternately in Bed 1 and Bed 2. To improve energy efficiency, in practice systems with two or more beds (with even numbers) are normally used.
Objective:
Adsorption desalination (AD) is a phase transfer process that uses the adsorption and desorption of water vapor by certain solid substances with porous adsorbents properties to achieve desalination.
Type of water
Seawater.
Brackish water.
High salinity brine.
Applications:
Seawater desalination.
Brackish water desalination.
Zero Liquid Discharge.
Comparison to established technologies:
Advantages:
Process driven by low grade thermal energy at lower temperature
Ability to utilize solar heat or low-grade waste energy at a temperature lower than 100ºC (50-85ºC)
Few moving parts (low pressures) and low temperatures: easy maintenance and standard materials.
Low fouling and corrosion: few pre-treatment requirements.
Opportunity to use commonly available environment-friendly adsorbent
Applicable for high salinity desalination.
Potential Zero Liquid Discharge applications and brine valuation.
Disadvantages:
Initial development stage
Applicability
TRL 3-4
Technological readiness level (TRL), it is an indicator of maturity of a particular technology. TRL is measured on a scale from 1 to 9, 1 being the lowest and 9 being the highest. A TRL of 9 reflects the implementation of an actual system in operational environment.
Reference:
J.C. Mankins, Technology Readiness Levels. White Paper, April, 1995 https://www.spacepropulsion.org/uploads/2/5/3/9/25392309/john_mankins_paper_of_4-6-95_trl.pdf
Other issues
- Porous materials
– Silica gel
– Zeolite
– MOFs (Metal organic frameworks)
– Active carbo
– Carbon nanotube
– Graphene
- Hybrid systems:
MED-AD (Multi-effect distillation and Adsorption cycle)
RO-AD (Reverse Osmosis and Adsorption)
Energy consumption:
Specific energy consumption, as reported in literature varies for different configurations, feed water concentrations, water recovery, salt rejection etc.
Seawater desalination:
The energy cost of AD plant includes the contributions of thermal energy and the electrical energy.
Related to the thermal energy consumed in the process, AD cycle utilizes only low temperature heat sources, typically less than 85 C, to produce potable water through the sorption processes. Being at low temperature, the heat source is deemed to be free because if it is unused, it would have been purged into the ambient. Such low temperature heat sources are available in abundance from either the industrial waste heat or the solar thermal energy source. For this reason, it is considered that, Thermal energy consumed= Free energy from waste heat.
The electrical energy consumption of the advanced AD cycle is computed to be about 1.38kWh/m3 whilst the conventional AD cycle consumes about 4.92kWh/m3.
References: K. Thu. Adsorption desalination: theory & experiments (2010).